HHC Legal Update — What UK Buyers Need to Know (2025)

HHC Legal Update — here’s the short version: the legal status of HHC in the UK is unsettled and under active review by regulators; meanwhile, sellers and buyers must also navigate new product-type rules (notably the single-use vape ban that came into force in 2025). This post summarises the current legal signals, what they mean for buyers and sellers, and practical steps to reduce legal and compliance risk.
Single-use (disposable) vape ban — effective 1 June 2025
A core change every buyer and seller needs to know: the UK banned the sale and supply of single-use (disposable) vapes from 1 June 2025. The ban covers non-rechargeable, single-use devices and applies across England, Wales, Scotland and Northern Ireland; refillable, rechargeable devices remain available but sellers should confirm compliance before listing disposables.
Why HHC’s legal status is unclear — PSA & ongoing ACMD review
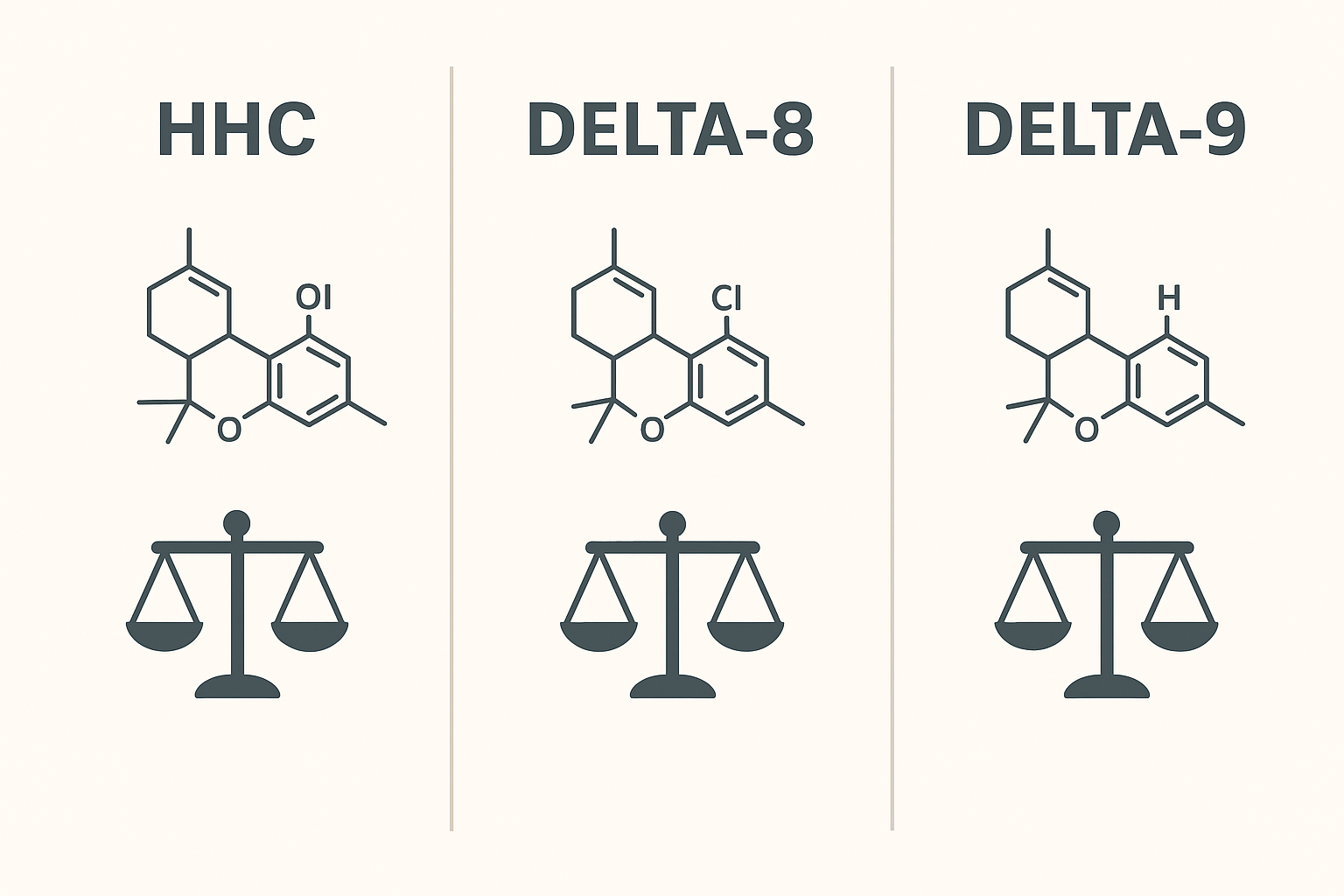
HHC is a semi-synthetic cannabinoid that has increasingly been detected in the UK market. Regulators are actively reviewing semi-synthetic and novel cannabinoids to determine whether and how they should be controlled. The UK’s Psychoactive Substances Act 2016 (PSA) provides the primary framework used to assess and, where appropriate, prohibit psychoactive substances — and recent ACMD work explicitly examines semi-synthetic cannabinoids (including HHC) as part of its programme. That means HHC may be subject to further controls or explicit guidance in future.
Practical implications for buyers (what this means for you)
-
Check whether the product is disposable or rechargeable. Disposable (single-use) vapes can be restricted or illegal to sell — prefer rechargeable, refillable devices if availability and compliance are a concern.
-
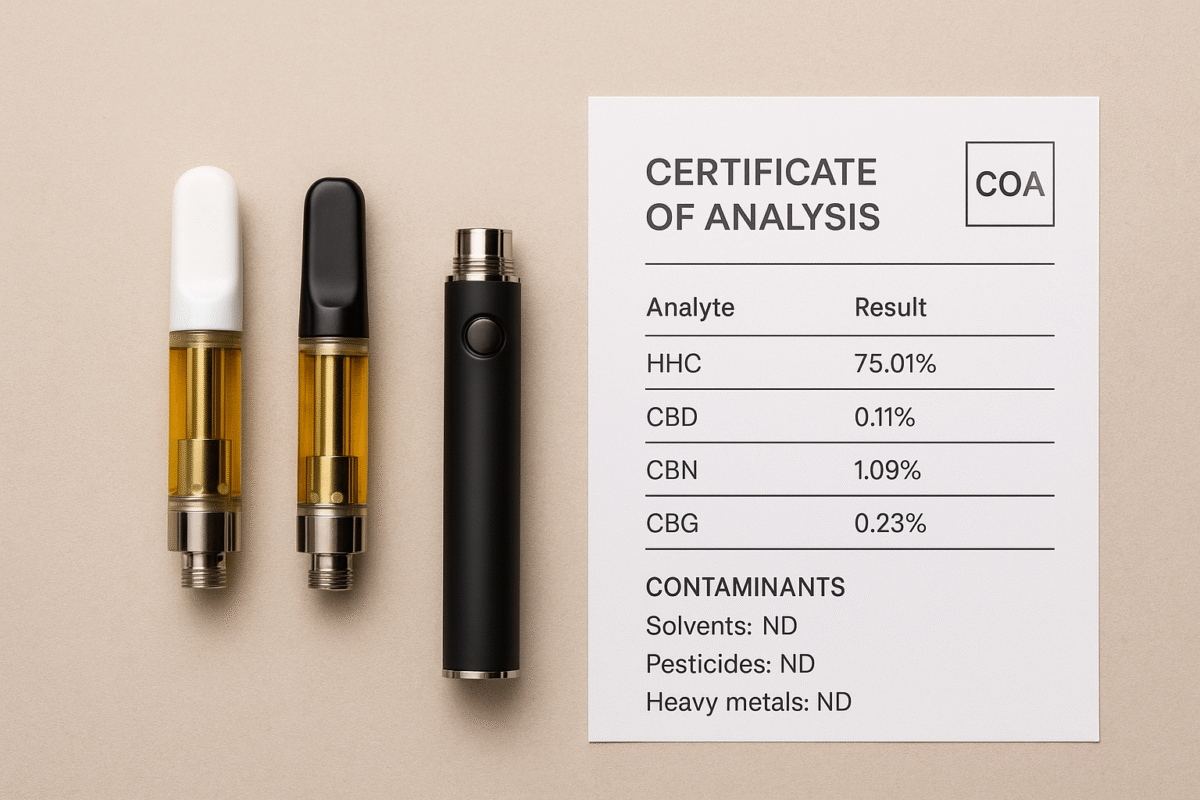
Insist on batch-specific COAs. Because regulatory attention is focused on novel cannabinoids and product safety, third-party Certificates of Analysis (COAs) are more important than ever — they show what’s in the product and whether contaminants are present. (See our COA guidance and how-to post for details.)
-
Understand that legal risk can be local and dynamic. The PSA/CPS gives prosecutorial authorities powers to act where a substance meets the definition of “psychoactive” — this means sellers and buyers face uncertainty until regulators publish final determinations.
Practical implications for sellers / site owners
-
Legal sign-off before listing or promoting lab-verified HHC range. Get written advice from legal/compliance counsel about selling or advertising HHC in the UK; regulators (and prosecutors) increasingly scrutinise novel cannabinoids and product-type rules (e.g., disposable vape ban).
-
Publish COAs and batch numbers prominently. Transparency reduces buyer risk and demonstrates good-faith compliance to regulators and platforms.
-
Keep product types compliant (no disposables unless clearly allowed). If you sell devices, prioritise rechargeable/reusable hardware and clearly label device type.
-
Age verification & robust terms. Use strict age checks at checkout, clear returns and shipping policies, and documented processes for handling compliance queries.
How regulators are approaching semi-synthetic cannabinoids (why the ACMD matters)
The Advisory Council on the Misuse of Drugs (ACMD) has been asked to review semi-synthetic cannabinoids and other emerging substances and to advise the Home Office on risk and appropriate control options. That ongoing work is a signal that government policy for substances like HHC may change — possibly swiftly if harms are identified or international scheduling shifts. Sellers should track ACMD outputs and Home Office guidance.
Short checklist for cautious buyers (actionable)
-
Confirm device type: Avoid single-use/disposable devices unless seller confirms compliance with the 2025 rules.
-
Request COA and batch number: Match the COA to the product batch before purchasing.
-
Ask about sourcing & processing: Prefer products where the vendor discloses source hemp, conversion steps, and solvents used.
-
Keep records: Save COAs, receipts and seller communications — useful if regulators or platforms query a transaction.
-
When in doubt, seek counsel: For high-volume sellers or distributors, get specific legal advice before listing or shipping HHC products.
What to watch next (regulatory signals & dates to track)
-
ACMD reports & commissioned work: ACMD publications and the Home Office work programme give the clearest early signals about control recommendations. Monitor GOV.UK for ACMD releases.
-
Home Office / GOV.UK guidance updates: The government will publish implementation details if it decides on specific controls.
-
CPS (Crown Prosecution Service) guidance & enforcement updates: Prosecutorial guidance under the PSA may be updates — monitor the Crown Prosecution Service for legal interpretation and enforcement notes.
Final thoughts & recommended next steps
HHC sits in a regulatory grey zone in 2025: it’s under scrutiny, and product-type rules (like the disposable vape ban) already affect how devices are sold. For buyers: prioritise transparency (COAs & batch IDs), prefer rechargeable hardware, and keep records of purchases. For sellers: secure legal sign-off, publish COAs, and ensure device types comply with the 2025 disposable-vape rules. If you want, we can draft a short compliance checklist for your product pages (COA placement, batch ID format, and mandatory disclaimer language) or a monitoring plan that alerts you when ACMD/Home Office pages update.
Related topics for HHC Legal Update
Understanding HHC & UK Regulations — Plain English Summary
HHC vs Delta-8 & Delta-9 — A Neutral Comparison for UK Buyers

 Weed Strains
Weed Strains Hybrid
Hybrid Indica
Indica Vapes
Vapes THC Vapes
THC Vapes Vape Cartridge
Vape Cartridge Disposable Vapes
Disposable Vapes Rechargeable Vapes
Rechargeable Vapes CBD Disposable Vape
CBD Disposable Vape Delta Vapes
Delta Vapes Delta 8 Vape
Delta 8 Vape Delta 9 Vape
Delta 9 Vape smart vape
smart vape Gummies
Gummies CBD Gummies
CBD Gummies THC Gummies
THC Gummies Delta 8 Gummies
Delta 8 Gummies Delta-9 Gummies
Delta-9 Gummies Delta-10 Gummies
Delta-10 Gummies HHC Gummies
HHC Gummies Full Spectrum Gummies
Full Spectrum Gummies Live Resin Gummies
Live Resin Gummies