What’s Inside a COA? Reading Lab Reports for Cartridges

COA lab report guide — a short, practical walkthrough so you can spot the important sections of a Certificate of Analysis and use that info to buy more safely. If you’re checking to shop HHC cartridges, this guide shows exactly what to look for on a COA, what the numbers mean, and which red flags should make you pause. (If you want a curated set of lab-verified cartridges to compare, you can shop HHC cartridges or browse our HHC collection
Why COAs matter
A COA is an independent lab’s report of what was detected in a specific product sample. For cartridge buyers, COAs are valuable because they confirm cannabinoid content, expose contaminants, and show when and where testing was done. Think of a COA as the product’s lab passport — it tells you what’s inside that particular batch.
1. Header & identification: confirm the basics first
The top of a COA usually includes administrative information. Verify these items before you read results:
-
Lab name & accreditation: Who performed the test? Accredited labs are more trustworthy.
-
Report date: How recent is the test? Prefer reports within 12 months for cannabis-derived products.
-
Sample ID / Batch number / SKU: The COA should reference the exact batch or SKU listed on the product page. If it doesn’t, don’t assume the COA matches the item you plan to buy.
-
Client or product name: Some COAs list the manufacturer or seller — check that it aligns with the product listing.
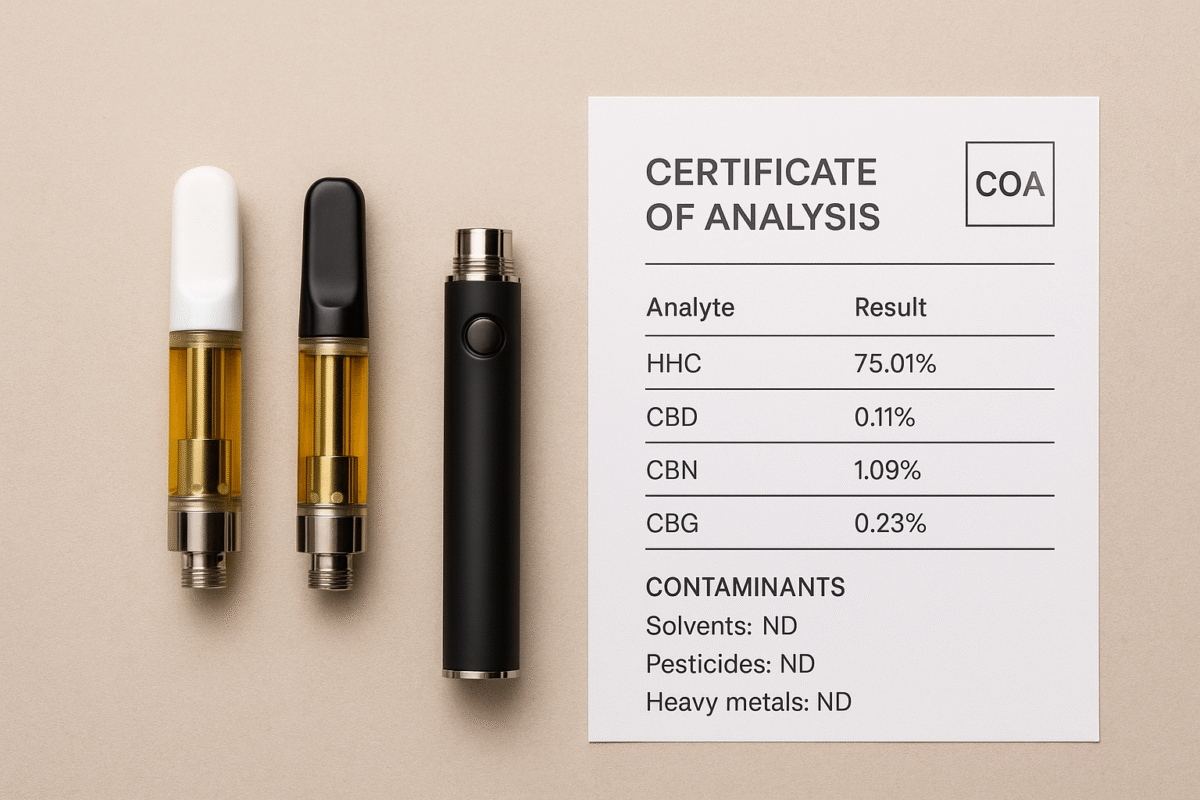
2. Cannabinoid panel: the “what’s in it” section
This is often the first results table you’ll see. It should list individual cannabinoids with concentrations (mg/g or %).
Key items to check:
-
Reported cannabinoids: Look for entries such as HHC, delta 8 uk, delta-9, CBD, CBG, etc. The primary compound expected should be present at a reasonable concentration.
-
Units: Labs commonly report % w/w or mg/g — know which one you’re reading.
-
Total THC reporting: Some COAs show total THC equivalents; if delta-9 is present in significant amounts that may affect legality depending on jurisdiction.
-
LOD/LOQ values: Limits of Detection/Quantification tell you how sensitive the test is — a “Not Detected” result is only meaningful relative to the LOQ.
3. Contaminant screens: safety-critical checks
This section is essential for converted or processed cannabinoids where solvents or pesticides may be present.
Typical contaminant panels include:
-
Residual solvents: Shows whether solvents used in extraction or conversion remain. Solvent residues should be “Not Detected” or under accepted limits.
-
Pesticides: A full-screen panel is ideal; missing pesticide testing is a red flag.
-
Heavy metals: Lead, cadmium, arsenic — any detections should be examined and contextualised against limits.
-
Microbial & mycotoxin tests: Especially for botanical extracts — ensures no harmful microbes are present.
4. Terpenes & additives (optional but helpful)
Some COAs include a terpene profile or list of additives (flavourings, carrier oils). This helps confirm if the product contains expected terpenes or unexpected flavouring chemicals.
5. Methodology & testing notes
A responsible COA will state the analytical methods used (e.g., GC-MS, HPLC) and any sample preparation notes. This helps you assess whether the test is appropriate for the claim (e.g., certain methods are better for volatile solvents, others for cannabinoids).
6. Certificate conclusion & signatures
Toward the end, look for an authorised signature and any lab disclaimers. Labs often include a statement about the sample’s representativeness — remember that the COA represents the tested sample, not every item off the production line unless batch control is strong.
Red flags to watch for
-
No batch number on the COA or mismatch to the product’s packaging.
-
Old report dates (several years old) — prefer recent testing.
-
Missing contaminant panels — e.g., no solvents or pesticide testing for processed products.
-
Non-accredited lab name with no methods listed — this reduces confidence in results.
-
Results that look rounded or suspiciously clean (e.g., all “Not Detected” across the board with no LOQ shown) — ask for method details or a different lab’s report.
How to verify a COA quickly (practical steps)
-
Match batch IDs: Confirm the COA’s batch/SKU matches the product page and product packaging.
-
Check the lab: Google the lab name and confirm accreditation or lab reports. Accredited labs or those with clear methodologies are preferable.
-
Scan for contaminants: Ensure solvents, pesticides and metals were tested and are within acceptable limits.
-
Save records: Download and store COAs linked to each purchase — they help if you later need to verify or raise an issue.
-
Ask the seller: If anything is unclear, request clarification or a second COA — legitimate sellers will respond.
A short buyer checklist (copyable)
-
COA present and downloadable ✔️
-
Batch number on COA matches product ✔️
-
Cannabinoid panel lists expected compound (e.g., HHC) ✔️
-
Contaminant screens include solvents, pesticides, heavy metals ✔️
-
Lab name & method listed; accreditation preferred ✔️
When to be extra cautious
Products made via chemical conversion (e.g., converted from CBD to other cannabinoids) or hydrogenation require extra scrutiny for residual solvents and byproducts. Always insist on full contaminant panels and clear method reporting.
Where to compare verified products quickly
If you’d rather start with a curated set that publishes COAs on product pages, consider our HHC collection or shop HHC cartridges
Final thoughts
A COA is the single most useful document for assessing a cartridge’s safety and content. Learn to read the major sections, insist on batch matching, and don’t buy from vendors that refuse to share COAs. With these checks you’ll make better, safer choices when shopping cartridges.
Related topics for COA lab report guide;
Where to Find Lab-Tested Cartridges in the UK (Red Flags to Avoid)
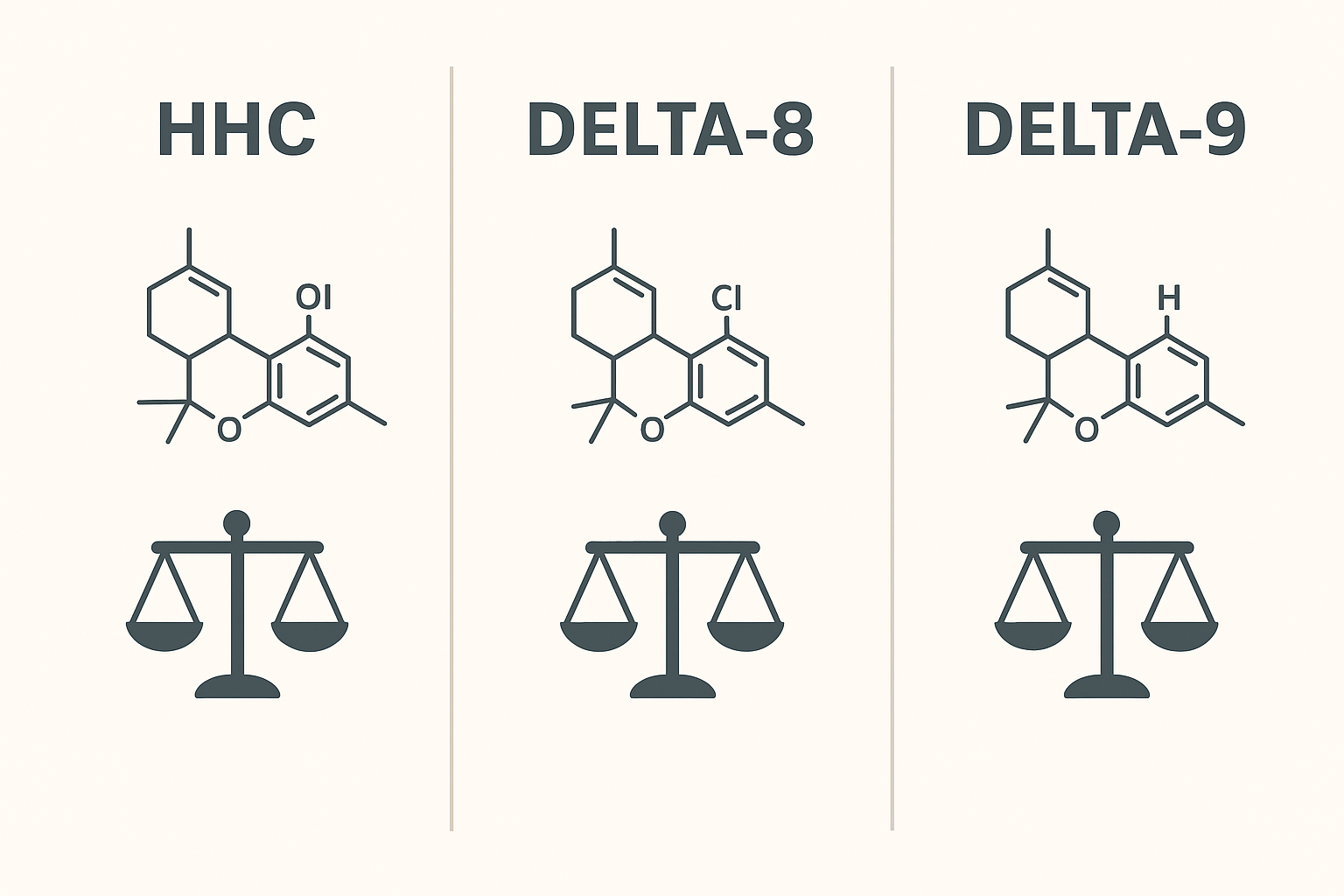
HHC vs Delta-8 & Delta-9 — A Neutral Comparison for UK Buyers
HHC Safety Basics: Storage, Batteries & Best Practices

 Weed Strains
Weed Strains Hybrid
Hybrid Indica
Indica Vapes
Vapes THC Vapes
THC Vapes Vape Cartridge
Vape Cartridge Disposable Vapes
Disposable Vapes Rechargeable Vapes
Rechargeable Vapes CBD Disposable Vape
CBD Disposable Vape Delta Vapes
Delta Vapes Delta 8 Vape
Delta 8 Vape Delta 9 Vape
Delta 9 Vape smart vape
smart vape Gummies
Gummies CBD Gummies
CBD Gummies THC Gummies
THC Gummies Delta 8 Gummies
Delta 8 Gummies Delta-9 Gummies
Delta-9 Gummies Delta-10 Gummies
Delta-10 Gummies HHC Gummies
HHC Gummies Full Spectrum Gummies
Full Spectrum Gummies Live Resin Gummies
Live Resin Gummies